Where will medical affairs create the most industry value?
Through our engagement with clients, new business consultations, and SMEs, we at Intuition are constantly learning about how Medical Affairs (MA) continues to evolve and refine the industry’s approach to some of its most critical scientific manufacturing and communication processes.
A recent publication by GOLD highlighted MA’s subtle influence and the historically misunderstood value that MA as a discipline has had to offer to the pharmaceutical engine.
MA once played the simple role of helping commercial teams introduce their product to market. This represented a final filter of sorts – quality checking promotional materials and ensuring reps were equipped with all the necessary product information to succeed. But as the scope of scientific knowledge has grown, so too has the responsibility of MA to grow with it. MA now represents itself across all areas of the organization, enriching the pharmaceutical and med-device value chain.
[Medical affairs transformation – Embracing the power of technology]

Research
We canvassed a random sample of industry professionals, postgraduate students, and thought leaders for their opinions on the growing role of MA within Life-Sciences.
Our question was simple:
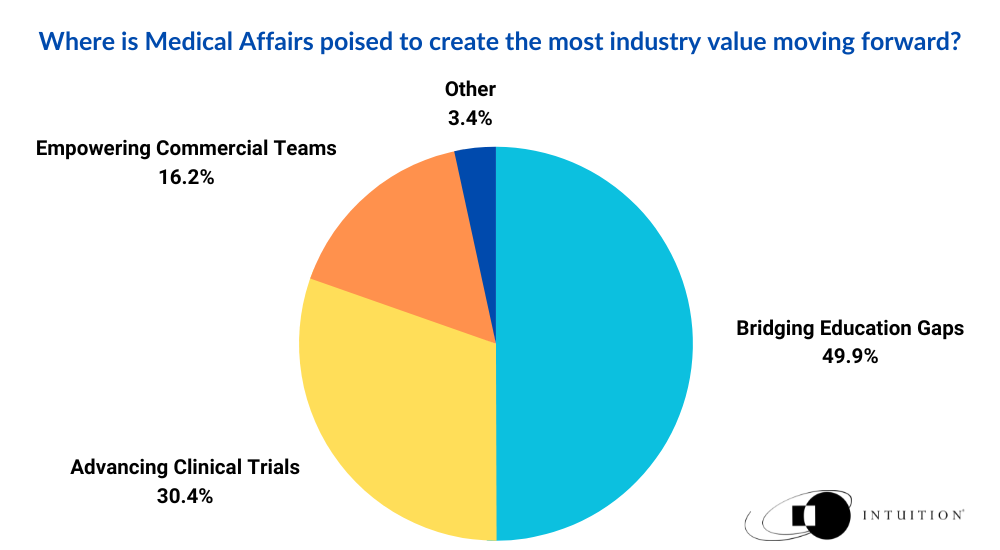
Where is Medical Affairs poised to create the most industry value moving forward?
- Bridging Education Gaps
- Empowering Commercial Teams
- Advancing Clinical Trials
- Other
While the parameters for a poll like this are relatively simple, the conversation it sparks is both vital and complex. The engagement we received off the back of this poll stemming from comments and direct outreach revealed a real appetite for this kind of discussion.
The caliber of respondents is also worth mentioning here. Among some of its contributors, the poll was engaged with by Heads of Market Access, Chief Scientific Officers, Directors of Global Medical Education, MDs, PhDs, CEOs and many more. We found this research did more to reflect industry demographics than anything we have since found in the market
Results
The results were even more interesting than the fact that the respondents were people of influence.
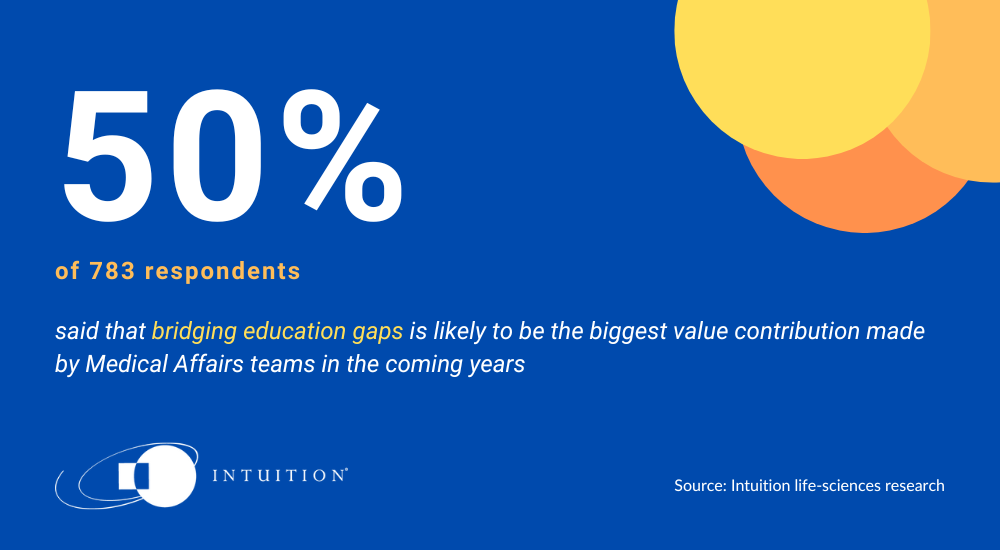
Of the 783 people polled, 50 percent of respondents chose Option 1, opining that ‘bridging education gaps’ is likely to be the biggest value add of MA teams in the future. This was interesting as it largely reflects the historical case made for MA as a function; helping communicate the inaccessible, scientific minutia of research findings into easily digestible, actionable points of summary.
The second most popular answer, with a share of 30 percent, was Option 3, ‘advancing clinical trials’. This is a growing business case for MA, as professionals aim to modernize their contribution to different aspects of the drug development life cycle, helping to improve in such areas as market access, discovery, and clinical research. This is reflected in the shifting industry narrative that is beginning to tell a much more compelling story for the use case of the department. MA is now breaking the glass ceiling of value demonstration, pivoting toward real-world evidence (RWE) generation, patient advocacy, and new means of external stakeholder engagement. It is a hugely exciting time for the function, which is partly why the popularity of this answer came as no surprise.
Option 2, ’Empowering commercial teams’ came in with 16 percent of the remaining vote, with Option 4 ‘other’ making up the spare change proportion of respondents, finishing with a total of 4 percent.
Conclusion
The MA function is evolving at pace, having finally broken free of years of pigeonholing at the hand of industry conservatism. Now, as organizations begin to reconsider the involvement of the function across departmental lines, we can begin to look toward how this value is being represented at scale.


