Medical affairs transformation – The power of technology
The past three years have reshaped perspectives on the pharmaceutical industry and the real-time adaptability that underpins its clinical processes.
Testament to the durability of the scientific method, the efforts of private interest, and a handful of key pharmaceutical stakeholders, the industry has paved the way in returning us to some semblance of normality after a challenging last few years.
The onus on the industry to react, adapt, and innovate over the past three years has pushed us toward new and unfamiliar scientific boundaries, the cost of which can come in the form of large-scale misunderstanding and – worse yet – public distrust.
These are not easy concepts to communicate; issues such as vaccine production, pharmaceutical economies of scale, or what we understand the long-term societal effects of the COVID-19 pandemic to be, remain challenging.
It all boils down to how and where we can afford to minimize translation error, and this is where medical affairs (MA) plays a key role.

The evolving role of medical affairs
Consider medical affairs professionals (MA) as mediators to the Life Sciences space, or as filters between front-end consumers and those at the cutting edge of research. They help communicate accurate information to healthcare providers and patient groups around some of the following issues:
- Publications
- Safety information
- Specifics about off-label usage
- Aspects of independent medical education
The medical affairs function has emerged to play a key role in how consumers experience medical information first hand. Two trends in particular are playing an increasingly significant role in shaping how medical information is being delivered.
The proliferation of data has bolstered the industry’s understanding of how it can harness ‘idle’ intelligence to guide new standards of decision making. At the same time, it creates a feedback loop that creates a whole new set of challenges for regulators where use cases, distribution rights, and standards of data maintenance all require definition.
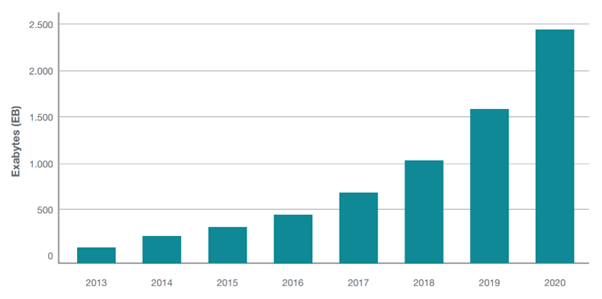
Figure 1: The growth of healthcare data 2013-2020, World Economic Forum
The emergence of patient-centred care has established itself as a running theme within the industry. The industry’s definition of value has begun to take account of new measures of clinical success and the evolving nature of the patient-physician dynamic.
[The future of Telehealth: From pandemic expansion to present-day adoption]
MA professionals are now tasked with looking at how to address these challenges in tandem: To grasp research insights and high-level discovery and in turn to make that understanding accessible to a much broader readership with an emphasis on the commercial-facing teams that rub shoulders with consumer groups. This kind of holistic work informs discussion from the top down – everything from policy decision making to casual, watercooler conversations.
The technology requirements are such that MA departments are equipped with the necessary tools to host and communicate educational materials, ultimately empowering understanding and improving patient outcomes. External-facing Learning Management Systems (LMS) have a significant role to play in this space moving forward.
Educating both sides of the conversation
Our healthcare systems are becoming increasingly resource constrained, even though the suite of available procedures continues to grow. HCPs need a measured understanding of where interventions sit both in terms of efficacy and financial viability.
MA professionals are tasked with setting the context and prepping the front window display for new technologies and novel alternatives as they make their way to market. But the buck does not stop with one’s local physician. Patients are more empowered than ever and can call a hard stop to being led down treatment pathways they do not want to visit.
Patient groups now play an equally executive role in the medical ecosystem. As the dynamics shift and the expectations of core consumers change, there is a more obvious stress placed on the provision of better educational infrastructure.
MA professionals are educating all segments of the population. They are communicating advances at both ends of the scale, helping the likes of vulnerable patient groups, physicians, and high-ranking pharmaceutical executives understand the comparative benefit of the newest available treatment alternatives.
The crucial plot point remains that education saves lives, improves process efficiency, and empowers our next step toward building a more resilient healthcare system. This is the level of intervention educational technology can play a role in.

Incorporating new models of engagement
Medical information is more complicated and specialized than ever, and it is estimated that our medical knowledge doubles every 73 days.
It is the job of MA to separate the wheat from the chaff in this climate of knowledge ‘noise’. It should look to communicate what is relevant and to silo what is not.
In order to scale this effort, MA departments will have to experiment with all kinds of new technologies positioned at reaching and crucially, engaging wider audiences. Built for purpose, an external-facing LMS can centralize groups of learners en masse, helping to streamline content access, facilitating cross collaboration and knowledge sharing to a whole new degree.
Promoting treatment awareness starts and ends with education. The challenge lies in knowing what technologies fit the design requirements. In terms of consolidating training requirements and leveraging new use-cases for advanced analytics, LMS providers might just be the silver bullet industry leaders have been searching for.
Conclusion
Real genius resides in the ability to distil complex ideas into elegant, engageable insights, accessible to all. This is the unforgiving faced by the MA function, which has become such a crucial pillar of innovation within the Life-Sciences space.
The dynamic will continue to ebb and flow in the coming years as the industry strikes a balance of responsibility between professionals and patient groups. The provision of better educational resources and the incorporation of more meaningful communication channels will only help to empower our collective understanding of where we are and where we are going.
Here at Intuition, we work in concert with the world’s largest pharmaceutical and biotechnology organizations to address these challenges. If you are interested in a product demo, feel free to get In touch, or sign up to our Life-Science’s focused newsletter, where we talk about some of the contributions we and our clients are making within this fascinating and ever-changing space.


