What is Pharmacovigilance?
Pharmacovigilance describes the various activities involved in the management and analysis of public health risks to ensure the rational use of medicines.
It involves the risk identification, quantification, and assessment associated with the use of medicines to avoid and minimize harm to patients.
Drugs that have made it to market have essentially run the pharmaceutical gauntlet, proving their efficacy and prescriptive value at every turn. Pharmacovigilance exists as a standardized method of measuring this value in new drug candidates. The effects of drugs are monitored at every stage of their life cycle, with new data constantly being collected and analysed.
As the regulatory landscape becomes increasingly stringent, pharmaceutical companies are forced to establish robust quality protocols. This provides strong incentives for these companies to ensure they maintain efficient systems for drug safety/ pharmacovigilance and making sure that all staff are aware of these basic requirements.
To help organizations stay in line with regulations, they follow the guidelines of Good Pharmacovigilance Practice (GVP).
What is good pharmacovigilance practice?
Good Pharmacovigilance practices (GVP) are a set of measures drawn up to facilitate the performance of pharmacovigilance in the European Union (EU). GVP apply to marketing-authorisation holders, the European Medicines Agency (EMA), and medicines regulatory authorities in EU Member States.
The purpose of these guidelines is to reduce the possibility of damage and harm caused by adverse drug events.

Where training comes into play
Training is vital for GVP. Each member of an organization that is intrinsically linked to the production of a drug/product must be appropriately qualified and sufficiently trained. The EMA provides draft guidelines on the topic, outlining what it means for employees to be appropriately trained under the banner of GVP.
These guidelines state that:
- All personnel involved must perform initial and continued training relating to their roles and responsibilities.
- All training must be assessed and monitored.
- All training content must be readily available to personnel should they become aware of a safety concern.
- There should be a process in place where the training results are monitored, and a satisfactory level of competency is met.
- Individuals who are not directly involved with the production of a drug should also be considered for training.
These guidelines also mention electronic systems, of which we are familiar with from 21 CFR part 11; each electronic system that holds documentation belonging to the organization must be validated. This includes Learning Management Systems – used to provide training in adverse events, patient safety, and other pharmacovigilance issues.
Benefits of training
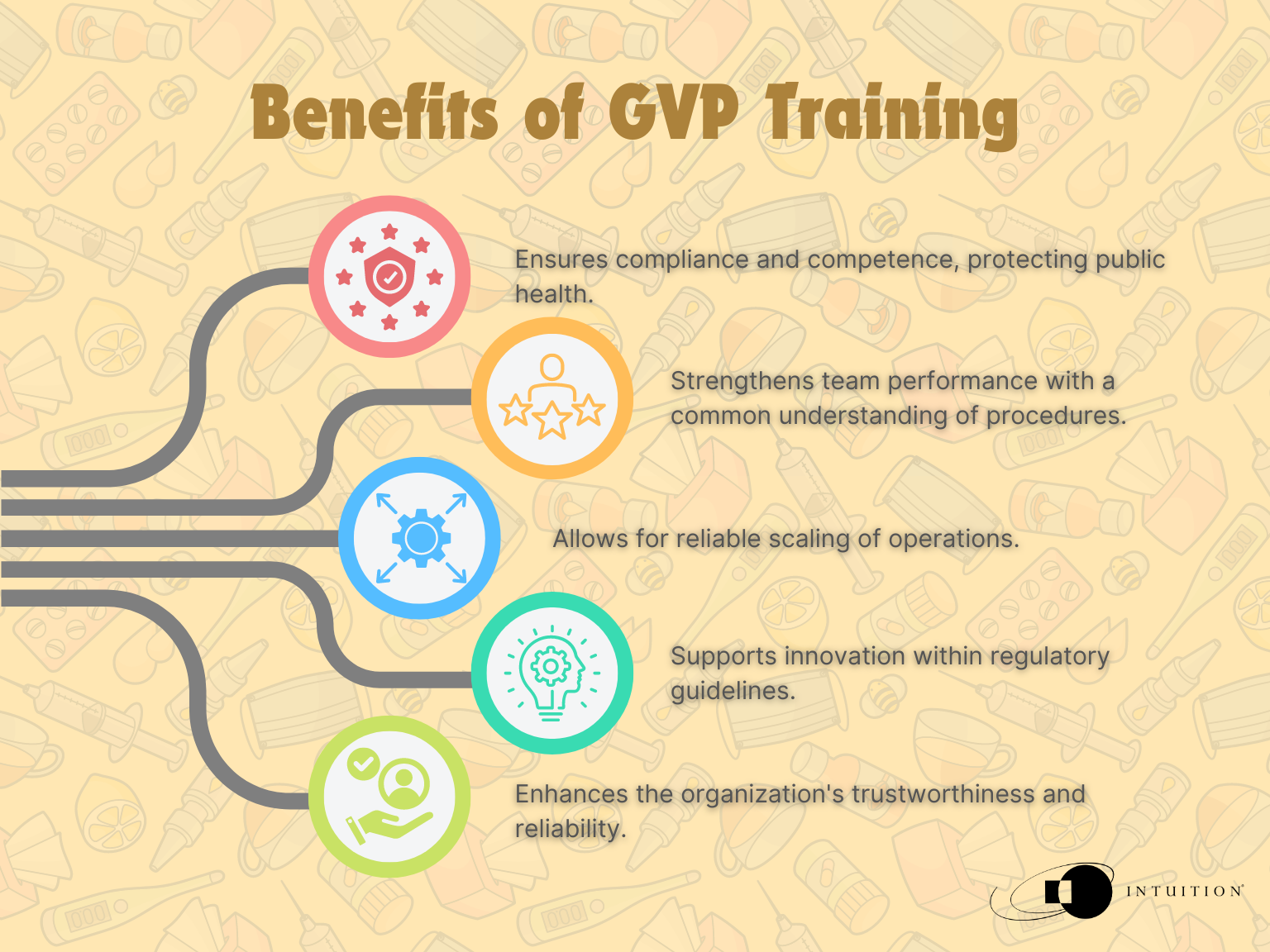
Maintaining these training requirements benefits the organization through compliance and competence and ensures that public health is protected.
Having a solid training system will help strengthen an entire team’s performance. Only with a common understanding for procedures, expectations, and processes can an organization reliably scale its operations. GVP training underpins this ambition, allowing companies to innovate within the appropriate regulatory guidelines to ensure the protection of patient safety standards across the industry.
The entire organization will gain from having a rock-solid foundation of knowledge creating a healthy culture of innovation and quality, not to mention from an outsider’s point of view, the organization would appear more trustworthy and reliable.
Conclusion
The dynamic will continue to ebb and flow in the coming years as the industry strikes a balance of responsibility between professionals and patient groups. The provision of better educational resources and the incorporation of more meaningful communication channels will only help to empower our collective understanding of where we are and where we are going.
Here at Intuition, we work in concert with the world’s largest pharmaceutical and biotechnology organizations to address these and other challenges. If you are interested in a product demo, feel free to get in touch, or sign up to our Life-Science’s focused newsletter, where we talk about some of the contributions we and our clients are making within this fascinating and ever-changing space.

References
- Pharmacovigilance training at WHO
- Short and long-term impact of pharmacovigilance training on the pharmacovigilance knowledge of medical students
- Pharmacovigilance: A Complete Guide to Pharmacovigilance and Drug Safety Training
- Pharmacovigilance and Drug Safety Training Helps Assure Employee Compliance with Quality and Patient Safety Standards
- Good Pharmacovigilance Practice Training: Why is it important?
- Good pharmacovigilance practices (GVP)
- Guideline on good pharmacovigilance practices (GVP)

